As we have seen, the concentrations of H2 and H+ both contribute to the ORP measurement result. If the H+ concentration changes, the ORP will also change, even if we keep the H2 concentration constant. In our analysis of the relationship between H2, H+ and ORP, we saw that ORP is much more responsive to changes in pH than to changes in dissolved H2 levels. “Why is this true?” Here is a summary of our findings:
- 1
The pH scale expresses H+ concentrations as powers of 10.
In our previous discussion about pH, we showed the equation used to calculate it:

The pH scale expresses changes in H+ using powers of 10.
If the pH value increases by one unit, this means a tenfold decrease in the H+ ion concentration (101 = 10). This means that an increase in pH by two units means a hundred-fold decrease (102 = 100) and a change of three units means a thousand-fold decrease (103 =1000) of H+.
Because pH changes are accompanied by exponential changes in H+ concentration, seemingly small changes in pH can actually result in very significant changes in the actual H+ concentration. The pH of hydrogen water can easily range over 3 units of pH (even more if you consider hydrogen water with a pH below 7).
This means that the H+ concentration of different H2 waters can vary by a factor of 10.000 or more! Finally, note that pH is only a mathematical representation of the actual H+ concentration (note that pH is given without a unit). However, the ORP electrode responds to the actual H+ concentration and not to the pH value.
- 2
Concentrations of H2 are expressed using a linear scale. Unlike expressing H+ concentrations like H+ using a logarithmic scale (pH), this uses a linear scale in milligrams per liter (mg/L). This typically covers a much smaller concentration range than H+. Consequently, changes in H2 typically represent relatively small changes compared to the large changes possible in H+.
For example, if the H2 concentration were expressed as powers of ten like pH, our concentration range of 0,5 to 5 mg/L represented only a concentration change of a factor of one power of ten (a tenfold change). This means we don't even come close to the factor of 10.000 that is possible with pH.
It is important to note at this point that a change in H2 level of just 1 or 2 mg/L seems small and actually has an imperceptible impact on the ORP measurement. However, it is very significant in terms of therapeutic benefit. The ORP measurement is therefore not suitable for measuring this.
So, to measure small changes in H2 levels, another method is needed to measure differences in H2 concentration of just a few mg/L.
- 3
The Nernst equation performs an exponential mathematical operation on the term [H+]. If we look again at the hydrogen gas reaction equation along with the Nernst equation showing the hydrogen redox couple, we can see another reason for the large influence of pH on ORP measurement:
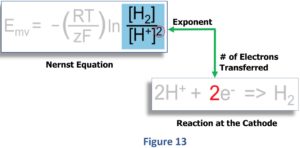
In the blue box of Figure 13, note that the lower term “H+]”, unlike the upper term [H2], contains the exponent “2” outside the parentheses (circled in red). Look at the equation below to see where this exponent comes from.
The Nernst equation now requires, due to the conversion of the oxidized species of the redox couple (2H+) to the reduced form (H2), that the number (2e-) of electrons (measured in “moles”) be expressed as an exponent for the term [H+]. becomes.
The explanation for this is not the subject of our discussion.
For reduction, which is by definition an enrichment with electrons, it makes sense that the amount of electrons required for the reduction reaction must be taken into account when calculating the reduction potential.
This exponent of “2” means we need to square the H+ concentration (multiply the [H+] term by itself).
Because the pH value indicates the amount of H+ on an exponential scale, the value of the H+ term is also the exponentially calculated H+ concentration! For the term [H2], however, there is no exponential function.
The exponential mathematical operation required by the Nernst equation helps explain how a seemingly small change in water pH (of just a few tenths) can result in such a large impact on the ORP measurement.
In comparison, the H2 concentration will only have a relatively insignificant influence on the ORP.
Taken together, points 1 to 3 clearly show why even seemingly small changes in pH represent exponentially larger changes in the actual H+ concentration. Consequently, the large influence of pH on the ORP measurement overcomes the small influence of H2 and essentially “controls” the ORP measurement.
Excerpt from the book by Randy Sharpe: “The relationship between dissolved H2, pH and redox potential”




