While there are many redox couples in chemistry, when discussing hydrogen water and ORP we are dealing with a specific redox couple, the redox couple H+/H2 4. Previously we showed the equation that can be used to describe the redox reaction in which two hydrogen ions (2H+ ) accept two electrons (2e-) to form a molecule of H2 gas.
Now in Equation 3 you can see the two species that make up the redox couple. “H+” is the oxidized form of hydrogen, which itself contains no electrons, and “H2” is the reduced form, which contains two electrons (see Figure 3). Since the H+ ion is a single proton that cannot donate electrons, it can only act as an oxidant and accept electrons.

Conversely, since the H2 molecule contains two electrons, it can (under the right conditions) donate them and act as a reducing (or antioxidant) agent. Later we will see how the simultaneous presence of H2 gas and H+ ions in water creates a negative ORP.
pH
As we will see later, the pH of the tested hydrogen water has a strong influence on the ORP measurement. Therefore, it is important to understand some basic concepts about pH.
Hydrogen ions are the acid component in water. The higher the H+ ion concentration, the more acidic the water is. The pH value, which stands for “potential of hydrogen” (Latin “potentia hydrogenii”), is a measure of the concentration of hydrogen ions (H+) in water. When considering atoms, molecules, ions, etc. in quantitative terms, the numbers can become extremely large or small.
The pH scale was therefore developed as a convenient way to express these types of numbers in powers of ten (exponents), rather than using equivalent (but more cumbersome) mathematical expressions such as 1×10-7 or 0,0000001.
The pH scale, which ranges from 0 to 14, is a logarithmic scale in which the concentration of H+ ions is expressed as a negative power of 10. Equation 4 is the equation for calculating the pH value.

Since pH is a logarithmic function, any change in pH of 1 represents a tenfold change in hydrogen ion concentration. As a result, very large changes in H+ concentration are expressed in powers of ten.
The presence of the minus sign in front of the “log” function allows pH to be expressed only in positive numbers for convenience, but also means that increases in pH represent decreases in concentration (and vice versa).
The name “hydrogen” can refer to one of four different ways:
2) H, hydrogen atom
4) H2, hydrogen molecule / molecular hydrogen
The term “pH” always refers to H+, the positive hydrogen ion.
A pH value of zero therefore contains a very high concentration of H+ ions, while a pH value of 14 has a very low H+ concentration. The “potential of hydrogen” is often mistakenly referred to as “the potential of hydrogen gas”. However, pH is not associated with dissolved H2 gas.
Adding pure H2 gas to water (e.g. through bubbling) does not change the pH of the water. However, certain processes for producing hydrogen water can indirectly increase the pH of the water.
For example, in a basic ionizer, during electrolysis of water (which reduces H+ ions to H2 gas at the cathode), the pH of the water is increased. However, the increase in pH is not caused by the presence of H2 gas. The real reason is the consumption of H+ ions (acid) and the increase in the content of OH- ions (base).
Recently, new hydrogen water technologies have been developed that aim to maximize dissolved hydrogen content while producing water with a pH close to neutral.
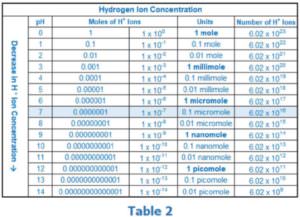
Table 2 shows the relationship between pH and hydrogen ion concentration. The concentration of H+ ions determines whether the water is acidic (below 7) or basic (above 7), with 7 representing a neutral pH. This table helps us see the exponential (logarithmic) relationship between pH and the actual number (concentration) of H+ ions. Since the H+ ion is one of the two species in the redox couple, it is not surprising that the pH of the water has a direct influence on the ORP measurement. In the next section we will introduce the Nernst equation, a mathematical tool that will help us evaluate this influence.
Excerpt from the book by Randy Sharpe: “The relationship between dissolved H2, pH and redox potential”




